Retinal Biologics Market Overvirew
The Retinal Biologics Market refers to the global industry involved in the development, manufacturing, and commercialization of biologic therapeutic agents specifically designed to treat retinal diseases. These biologics are complex molecules derived from living organisms—such as proteins, antibodies, and gene therapies—and are used to address retinal disorders including macular degeneration, diabetic retinopathy, uveitis, retinal tear, and retinitis pigmentosa.
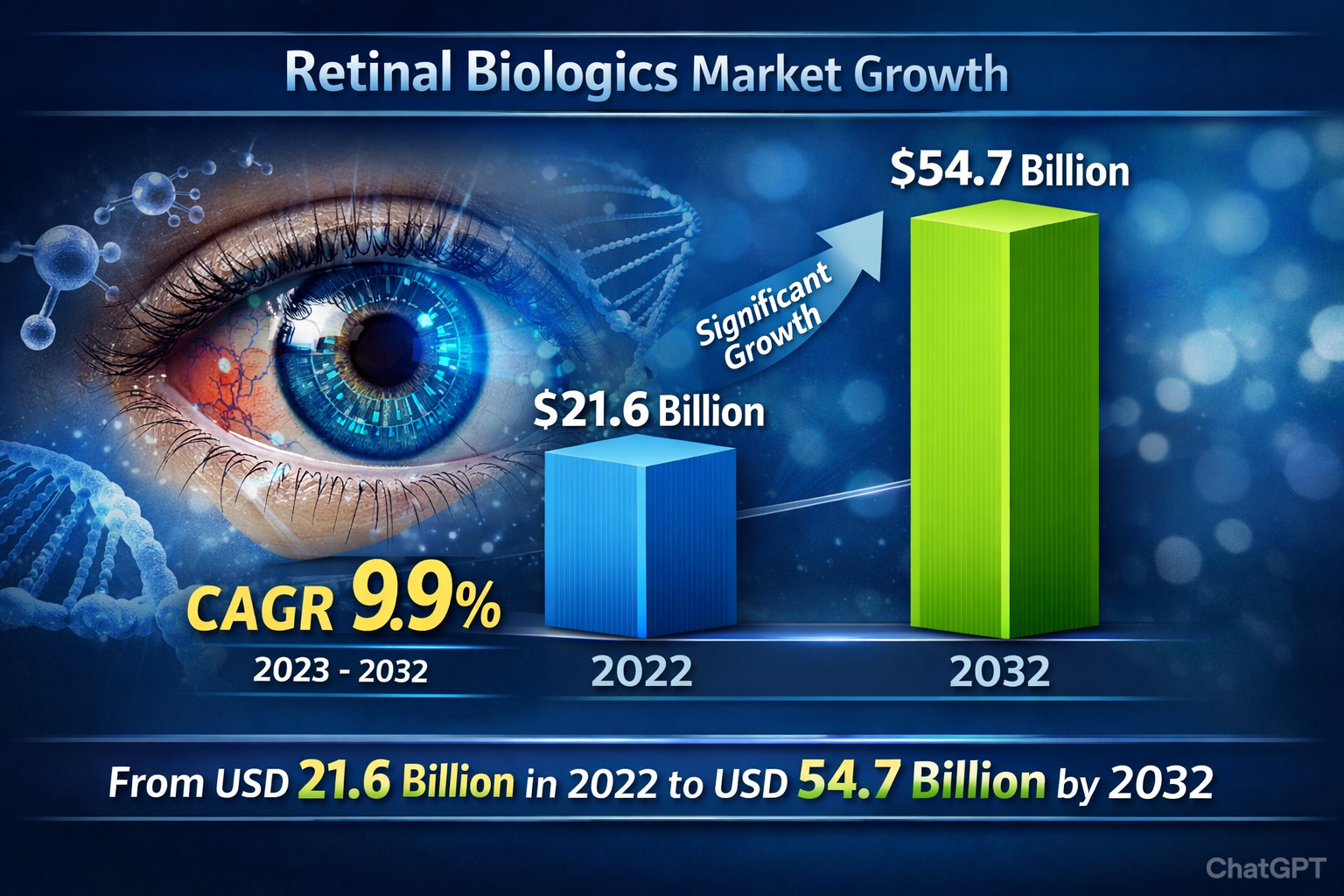
Retinal biologics play a vital role in slowing or reversing vision loss by targeting underlying biological pathways (like vascular endothelial growth factor – VEGF) that contribute to disease progression. The market was valued at approximately USD 21.6 billion in 2022 and is projected to grow to around USD 54.7 billion by 2032, at a CAGR of 9.9% between 2023 and 2032.
The significance of this market lies in:
Rapidly rising prevalence of retinal diseases globally, especially age‑related macular degeneration.
Advances in biologic therapies that offer higher efficacy and safer profiles than traditional small‑molecule drugs.
Growing demand from aging populations and improved healthcare awareness.
Get a Free Sample: https://www.acumenresearchandconsulting.com/request-sample/3542
Market Trends
The retinal biologics landscape is evolving rapidly due to several key trends:
Technological Advancements
Technological innovation continues to transform retinal therapies. New drug delivery systems and improved biologic molecules are enabling more effective and longer‑lasting treatments for vision‑related disorders. There’s also a rising focus on gene therapies and advanced biologic combinations targeting multiple disease pathways.
Growing Focus on Diagnostic Tools
Alongside biologics, diagnostic tools such as advanced imaging and biomarker panels are improving disease detection. These advancements help clinicians identify and treat retinal conditions earlier, driving higher adoption of effective biologic therapies.
Shift in Consumer Behavior
Patients and physicians are increasingly favoring biologic therapies due to enhanced efficacy and reduced side effects, compared to conventional drugs. Awareness campaigns about early detection and treatment of retinal diseases are also encouraging more frequent eye check‑ups, expanding the potential patient pool.
Expansion into Emerging Regions
Emerging markets, particularly in the Asia‑Pacific region, are gaining traction thanks to substantial healthcare investments and rising incidence of retinal illnesses. The region is expected to grow at a CAGR of more than 11%, outpacing other markets.
Market Dynamics
Understanding the forces that influence this market can illuminate its growth trajectory:
Growth Drivers
Rising prevalence of retinal diseases such as AMD and diabetic retinopathy.
Advances in biologic drug development and approvals, expanding treatment options.
Adoption of imaging innovations that support early diagnosis and treatment monitoring.
Restraints
High treatment costs and limited access in underdeveloped regions remain significant barriers.
The stringent regulatory approval processes and lengthy clinical trials slow new product entries.
Opportunities
Launch of next‑generation biologics with extended dosing intervals and improved safety profiles.
Rapid improvements in ocular drug delivery systems, increasing treatment uptake.
Growing healthcare infrastructure in regions like Latin America and Middle East & Africa present new markets for expansion.
Regional Analysis
The Retinal Biologics Market shows distinct regional trends driven by healthcare infrastructure, disease prevalence, and investment levels.
North America
North America remains the dominant market, accounting for a significant share of global revenue. This leadership is attributed to strong healthcare systems, extensive adoption of advanced therapies, and high R&D investment by major pharmaceutical companies. New product approvals and partnerships further strengthen its position.
Europe
Europe exhibits steady growth supported by strategic collaborations, funding for ophthalmic research, and increasing uptake of biologic drugs. Reimbursement frameworks and preventive health policies also play a crucial role in adoption rates.
Asia‑Pacific
With a large patient base and expanding healthcare networks, Asia‑Pacific is among the fastest‑growing regions. Increasing research funding, healthcare spending, and rising disease burden contribute to its high projected CAGR.
Latin America & Middle East/Africa
While growth is slower compared to developed markets, improved access to biologic therapies and ongoing awareness campaigns are enhancing market penetration. These regions represent emerging frontiers with potential for future expansion.
Recent Developments in the Market
Major Biotech Acquisition
In May 2024, Merck & Co. announced plans to acquire an eye-focused biotech company for up to USD 3 billion. This deal strengthens Merck’s portfolio in retinal disease drug development, notably involving a promising candidate targeting diabetic macular edema and neovascular AMD—two principal retinal conditions.
Increased Biologic Approvals
Regulatory bodies have continued to approve novel biologics for retinal diseases, including expanded indications and updated labeling based on safety and efficacy data.
Conclusion
The Retinal Biologics Market is experiencing robust expansion driven by rising prevalence of retinal disorders, technological innovation, and expanding global healthcare access. While cost and regulatory hurdles persist, opportunities in emerging economies and novel therapy development promise sustained long-term growth. With advancements in biologics and supporting diagnostics, the industry is well-positioned to revolutionize retina-focused patient care worldwide.
To Get Detailed Overview, Contact Us: https://www.acumenresearchandconsulting.com/contact-us