Multiple System Atrophy Therapeutics Market
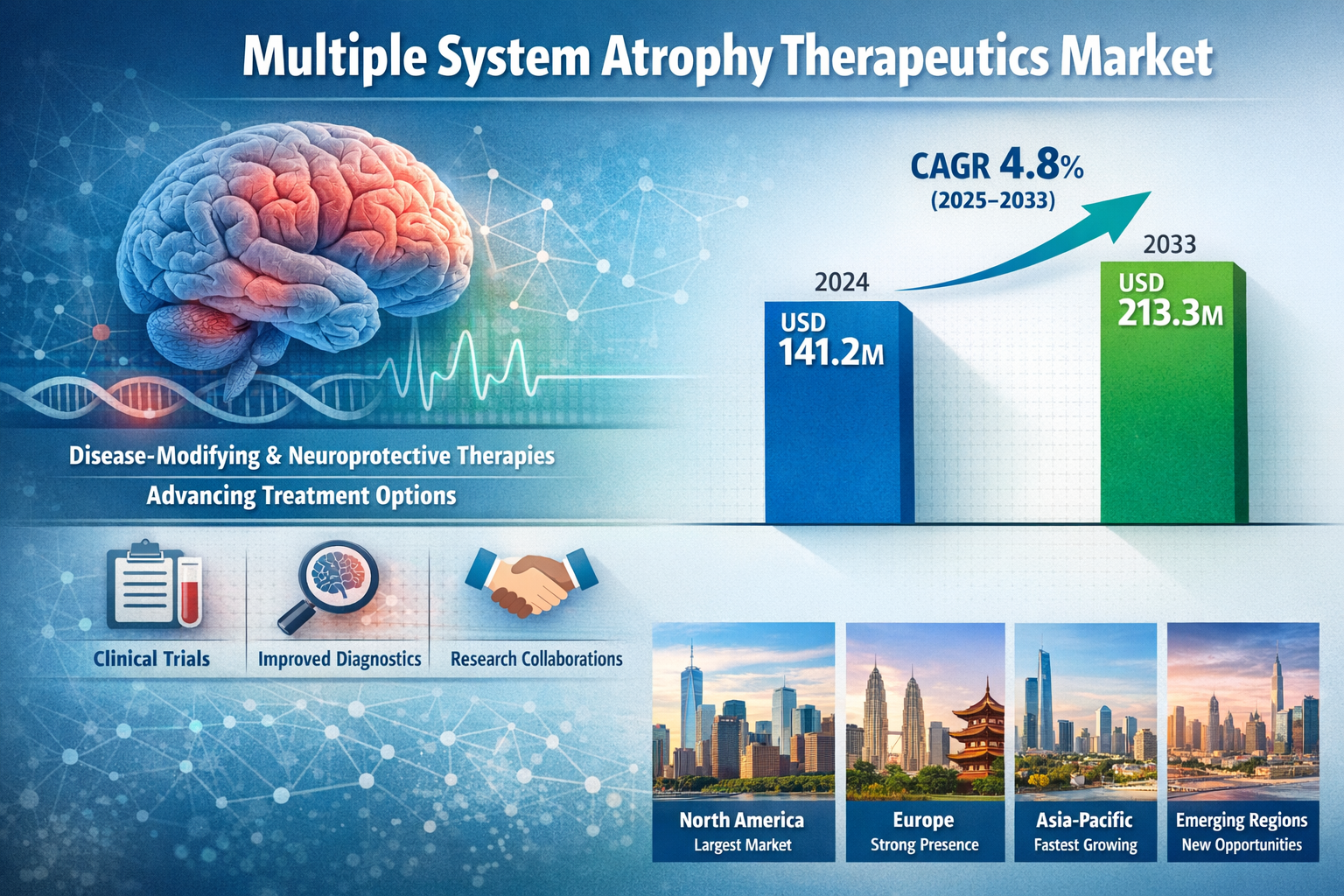
The global multiple system atrophy therapeutics market was valued at USD 141.2 Million in 2024 and is projected to reach USD 213.3 Million by 2033, growing at a CAGR of 4.8% during 2025–2033.
What is the Multiple System Atrophy Therapeutics Market?
The multiple system atrophy therapeutics market encompasses pharmaceutical drugs and treatment approaches designed to address the symptoms and underlying mechanisms of Multiple System Atrophy. These therapies primarily target motor dysfunction, autonomic instability, and neurodegenerative progression associated with the disease.
Currently, most available treatments focus on symptomatic relief, such as managing blood pressure abnormalities, muscle rigidity, and movement disorders. However, ongoing research is increasingly directed toward disease-modifying and neuroprotective therapies that aim to alter disease progression rather than only manage symptoms.
The significance of this market lies in its potential to address a rare but devastating disorder with limited therapeutic options. Increasing investment in orphan drug development, growing clinical trial activity, and rising neurological research funding highlight the strategic importance of this market within the broader neurodegenerative disease therapeutics landscape.
Get a Free Sample: https://www.acumenresearchandconsulting.com/request-sample/3776
Market Trends
The Multiple System Atrophy Therapeutics Market is evolving due to several notable trends:
Shift Toward Disease-Modifying Therapies
A major trend is the transition from purely symptomatic treatments to therapies that target underlying disease mechanisms such as alpha-synuclein aggregation, neuroinflammation, and neuronal degeneration. This shift reflects a broader movement within neurology toward precision and disease-altering interventions.
Advancements in Diagnostic Technologies
Improved diagnostic tools, including advanced neuroimaging and biomarker-based detection methods, are enhancing early and accurate diagnosis of MSA. Earlier diagnosis increases treatment initiation rates, thereby supporting market growth.
Increased Research Collaborations
Pharmaceutical companies are increasingly collaborating with academic institutions and biotechnology firms to accelerate drug discovery and clinical development. These collaborations help reduce development risks and improve innovation efficiency.
Pipeline Expansion and Drug Innovation
Several investigational therapies are progressing through clinical pipelines, targeting diverse pathways such as neuroprotection, oxidative stress reduction, and protein misfolding. The growing pipeline reflects heightened scientific interest and long-term growth potential.
Market Dynamics
Key Drivers
Rising Prevalence of Neurodegenerative Disorders: An aging global population is contributing to an increase in neurological diseases, including rare disorders like MSA.
Expansion of Research and Development Activities: Increased funding and research initiatives are accelerating therapeutic innovation.
Improved Disease Awareness and Diagnosis: Enhanced awareness among healthcare professionals is leading to improved diagnosis and treatment adoption.
Market Restraints
Limited Approved Treatment Options: The absence of curative or disease-modifying therapies restricts market expansion.
Diagnostic Complexity: Overlapping symptoms with Parkinson’s disease and other neurodegenerative disorders often delay accurate diagnosis.
High Development Costs: Drug development for rare diseases involves substantial financial and regulatory challenges.
Opportunities
Orphan Drug Development Incentives: Regulatory support for rare disease therapies offers financial and approval advantages.
Emerging Markets Expansion: Improving healthcare infrastructure in developing regions presents untapped growth potential.
Innovative Therapeutic Approaches: Breakthrough success in disease-modifying therapies could significantly transform market dynamics.
Challenges
Small Patient Population: Limited patient numbers make large-scale clinical trials challenging.
Complex Disease Biology: Incomplete understanding of MSA pathophysiology complicates drug development.
Regional Analysis
North America
North America holds the largest share of the Multiple System Atrophy Therapeutics Market. This dominance is driven by advanced healthcare infrastructure, strong neurological research ecosystems, higher disease awareness, and supportive regulatory frameworks for orphan drugs.
Europe
Europe represents a significant market due to well-established healthcare systems, active clinical research programs, and government support for rare disease treatment development.
Asia-Pacific
The Asia-Pacific region is expected to witness the fastest growth during the forecast period. Factors contributing to this growth include a rapidly aging population, improving access to healthcare services, increasing neurological research investment, and rising awareness of rare neurological disorders.
Latin America and Middle East & Africa
These regions are emerging markets with gradual growth driven by improving diagnostic capabilities, expanding healthcare access, and growing focus on neurological disease management.
Recent Developments
Recent developments in the Multiple System Atrophy Therapeutics Market reflect growing innovation and strategic focus:
Pharmaceutical and biotechnology companies are advancing novel compounds targeting neurodegenerative pathways associated with MSA.
Increased clinical trial activity is focused on evaluating disease-modifying and neuroprotective therapies.
Strategic collaborations between drug developers and research institutions are accelerating therapeutic discovery and development timelines.
Expanded investment in rare neurological disease research indicates strong long-term market confidence.
While no definitive cure has yet emerged, these developments signal meaningful progress toward more effective treatment options.
Conclusion
The Multiple System Atrophy Therapeutics Market is positioned for steady growth, driven by rising neurological disease burden, increasing R&D investments, and growing emphasis on disease-modifying therapies. Although challenges such as diagnostic complexity and limited treatment options remain, ongoing innovation, collaborative research, and supportive regulatory frameworks are expected to shape the future of this market. As therapeutic pipelines mature, the market holds promising potential to significantly improve outcomes for patients living with Multiple System Atrophy.
To Get Detailed Overview, Contact Us: https://www.acumenresearchandconsulting.com/contact-us