Table of Contents
ToggleClinical Trial Supply and Logistics Market Size
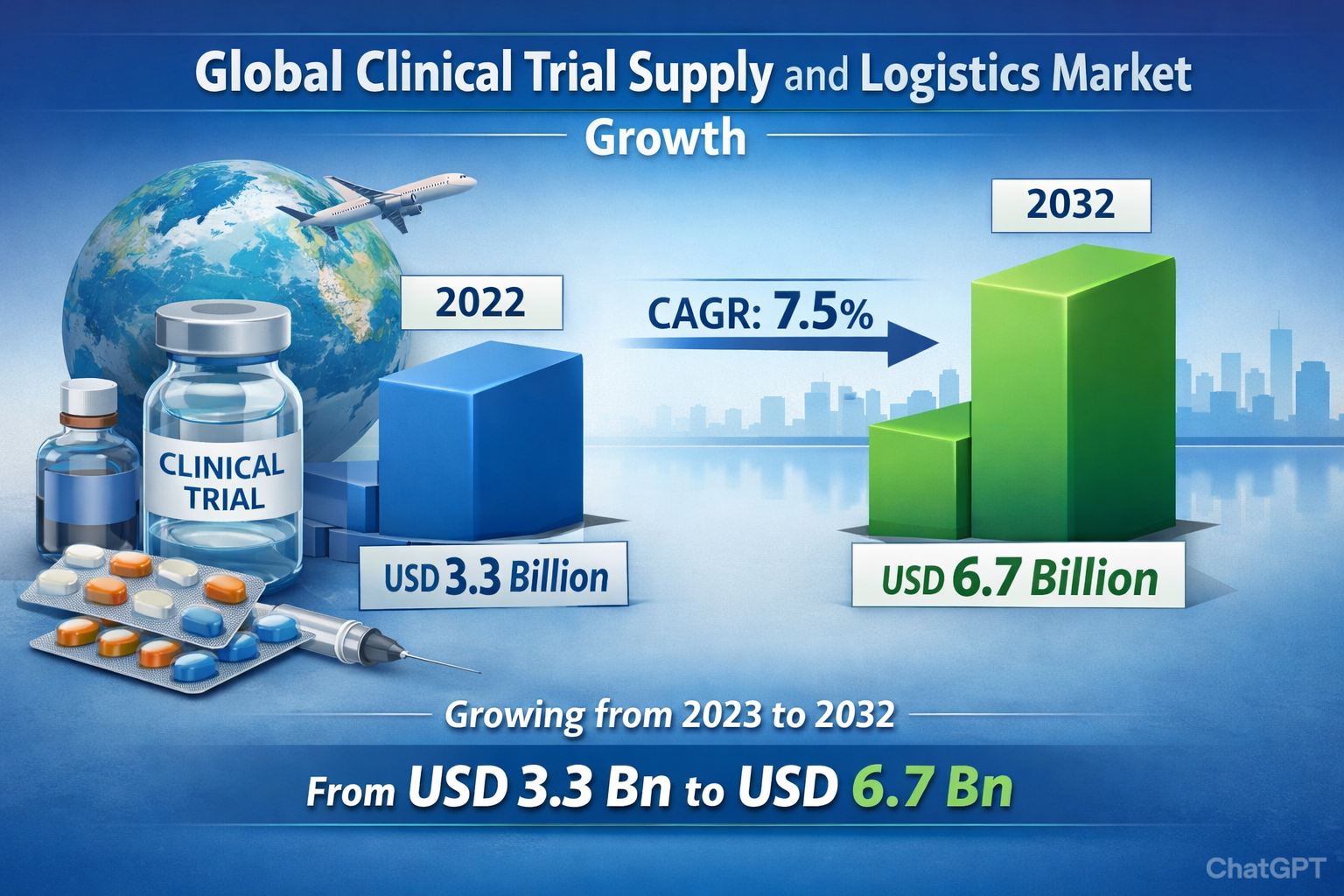
The global clinical trial supply and logistics market was valued at USD 3.3 billion in 2022 and is projected to reach USD 6.7 billion by 2032, exhibiting a strong compound annual growth rate (CAGR) of 7.5% over the forecast period.
What Is the Clinical Trial Supply and Logistics Market?
The Clinical Trial Supply and Logistics Market refers to the planning, management, and operational execution involved in delivering investigational products, medical devices, and associated materials required for clinical trials. Its purpose is to ensure that clinical supplies — including investigational drugs, comparators, and placebos — are manufactured, stored, packaged, labeled, transported, and delivered to clinical sites in a timely and compliant manner.
The market typically includes services such as:
Logistics and distribution
Packaging, labeling, and blinding
Storage and retention
Comparator sourcing
Manufacturing support
This market plays a critical role in clinical research because inefficiencies or disruptions in the supply chain can directly affect trial timelines, regulatory compliance, data integrity, and overall drug development outcomes.
Get a Free Sample: https://www.acumenresearchandconsulting.com/request-sample/3530
Market Trends
The clinical trial supply and logistics landscape is evolving rapidly as clinical research becomes more sophisticated and global.
Increasing Clinical Trial Complexity
Clinical trials are becoming more complex due to the rise of precision medicine, targeted therapies, and adaptive trial designs. These studies often involve multiple treatment arms, varying dosage regimens, and stricter protocol requirements, increasing the need for flexible and highly coordinated logistics systems.
Globalization of Clinical Trials
Clinical trials are increasingly conducted across multiple countries to accelerate patient recruitment and improve demographic diversity. This trend has intensified demand for cross-border logistics solutions capable of navigating regulatory variations, customs requirements, and regional infrastructure challenges.
Technology Integration in Supply Chains
Technological advancements are transforming clinical trial logistics. Digital platforms, predictive analytics, IoT-based monitoring, blockchain systems, and real-time tracking tools are improving visibility, traceability, and decision-making across the supply chain. These innovations help reduce wastage, minimize delays, and enhance operational efficiency.
Growth of Temperature-Sensitive Logistics
The increasing use of biologics, cell therapies, and gene therapies has elevated demand for temperature-controlled logistics. Maintaining strict environmental conditions during storage and transportation is now a central requirement, driving investment in advanced cold chain infrastructure.
Shift Toward Patient-Centric Trials
Decentralized and hybrid clinical trial models are gaining traction. These approaches often require direct-to-patient shipments, decentralized storage models, and more dynamic supply forecasting strategies, redefining traditional logistics frameworks.
Market Dynamics
Drivers
Rising Number of Clinical Trials
An expanding global pipeline of clinical trials, particularly in oncology, rare diseases, and specialty therapeutics, continues to drive demand for supply and logistics services.
Expansion of Global Research Networks
Sponsors increasingly conduct multi-regional studies, necessitating sophisticated logistics capabilities to support diverse trial locations.
Regulatory Compliance Requirements
Strict regulatory expectations regarding storage, labeling, transportation, and documentation have increased reliance on specialized logistics providers.
Restraints
Stringent Regulatory Frameworks
Differing regulatory requirements across countries can introduce delays, increase complexity, and elevate compliance costs.
High Operational Costs
Specialized packaging, cold chain management, and advanced tracking technologies contribute to higher logistical expenditures, particularly affecting smaller sponsors.
Opportunities
Adoption of Advanced Technologies
Digitalization, automation, and AI-driven forecasting present opportunities to enhance efficiency, reduce wastage, and improve supply accuracy.
Growth of Decentralized Trial Logistics
Emerging trial models create demand for innovative distribution approaches, including direct-to-patient delivery systems.
Challenges
Cold Chain Integrity
Ensuring consistent temperature control across global transportation networks remains a persistent challenge.
Multi-Site Supply Coordination
Managing supplies across numerous clinical sites requires robust planning systems, real-time visibility, and agile logistics strategies.
Regional Analysis
North America
North America has historically accounted for a significant share of the global clinical trial supply and logistics market. The region benefits from a high concentration of clinical trials, strong regulatory frameworks, advanced healthcare infrastructure, and the presence of major pharmaceutical and biotechnology companies.
Asia-Pacific
Asia-Pacific represents one of the fastest-growing regional markets. Growth is driven by expanding clinical research activity, large patient populations, improving regulatory processes, and increasing investments in pharmaceutical development. Countries such as India, China, and Japan play pivotal roles in regional expansion.
Europe
Europe maintains a stable presence in the market, supported by strong clinical research ecosystems, regulatory harmonization efforts, and a well-established network of clinical trial sites.
Latin America and Middle East & Africa
These regions are emerging as growth areas, driven by increasing clinical trial activity, expanding CRO presence, and growing interest from global sponsors seeking diversified trial locations.
Recent Developments
The clinical trial supply and logistics market has witnessed several notable developments:
Strategic Acquisitions and Expansions
Major logistics providers and healthcare supply companies continue to expand their capabilities through acquisitions and partnerships, particularly in temperature-controlled logistics and specialized courier services.
Strengthening of Cold Chain Infrastructure
Companies are investing heavily in advanced cold storage facilities, temperature-monitoring technologies, and specialized transportation systems to meet the needs of biologics and advanced therapies.
Integration of Digital Supply Chain Solutions
Increased adoption of digital platforms and analytics tools is improving supply chain visibility, demand forecasting, and risk management.
Growing Focus on End-to-End Service Models
Market participants are increasingly offering integrated solutions that combine packaging, storage, distribution, and trial management support under unified service frameworks.
Conclusion
The Clinical Trial Supply and Logistics Market is a foundational component of the clinical research ecosystem. As trials become more complex, global, and patient-centric, the need for sophisticated, technology-enabled, and regulatory-compliant logistics solutions continues to grow.
Organizations operating in pharmaceuticals, biotechnology, and clinical research must increasingly prioritize supply chain resilience, flexibility, and innovation. Efficient clinical trial logistics are no longer merely operational necessities — they are strategic enablers of faster, more reliable drug development.
To Get Detailed Overview, Contact Us: https://www.acumenresearchandconsulting.com/contact-us