Non‑Invasive Prenatal Testing Market Size
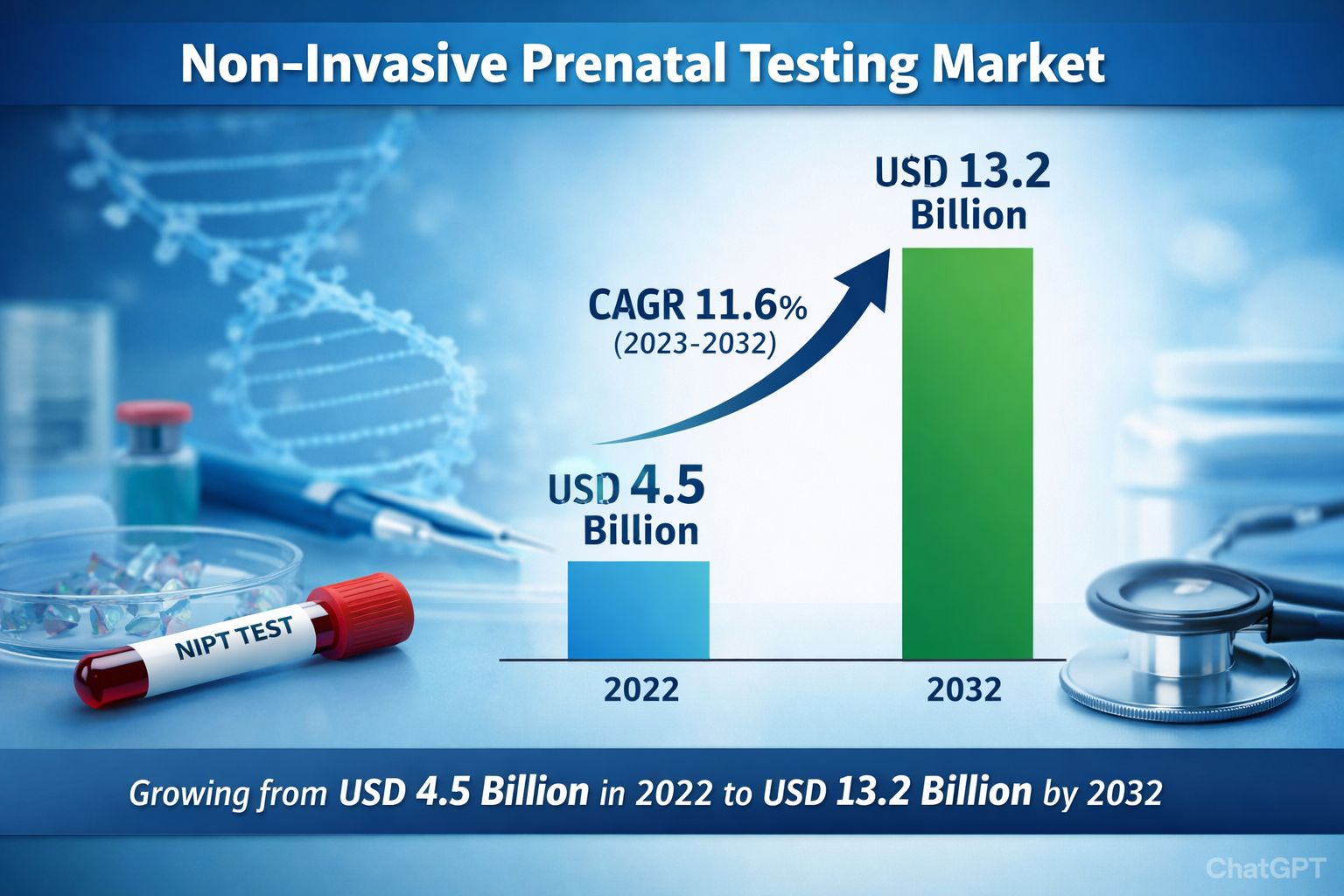
The global non-invasive prenatal testing market was valued at USD 4.5 billion in 2022 and is expected to expand to USD 13.2 billion by 2032, growing at a CAGR of 11.6% over the forecast period.
What Is the Non‑Invasive Prenatal Testing Market?
The Non‑Invasive Prenatal Testing Market refers to the global industry involved in the development, production, and adoption of tests that screen for fetal genetic abnormalities using a simple maternal blood sample. Unlike traditional invasive procedures such as amniocentesis or chorionic villus sampling, NIPT analyzes cell‑free fetal DNA (cfDNA) circulating in the mother’s bloodstream, offering safer and highly accurate detection of chromosomal disorders such as Down syndrome (trisomy 21), Edwards syndrome (trisomy 18), and Patau syndrome (trisomy 13).
Market Significance
Major market segments include kits & reagents, instruments, and services, with kits & reagents holding a leading share due to high utilization rates in testing procedures.
Applications range from common trisomy screening to emerging areas like sex chromosome and other complex genetic conditions.
NIPT’s increasing adoption is driven by its high specificity, convenience, and non‑invasive nature, making it essential in modern prenatal care.
Get a Free Sample: https://www.acumenresearchandconsulting.com/request-sample/3513
Market Trends
The NIPT market is shaped by several forward‑trending dynamics:
Technological Advancements
Continuous innovation in genetic sequencing and analytical methods has improved the accuracy and reliability of test results, enabling detection of a broader range of fetal genetic conditions.
Increased Awareness and Adoption
Growing awareness among patients and healthcare professionals, combined with an emphasis on early and precise fetal health assessment, has expanded test acceptance worldwide.
Changing Demographics
The trend toward delayed parenthood has increased the demand for early chromosomal abnormality detection—especially in women over age 35—fuelling market growth.
Expansion of Clinical Use
Hospitals and diagnostic labs are adopting NIPT as part of standard prenatal screening workflows, integrating it into routine maternity care due to patient safety and clinical accuracy benefits.
Market Dynamics
A successful snapshot of the key market forces:
Drivers
High demand for early genetic susceptibility detection, reducing the need for invasive procedures.
Technological improvements that enhance sensitivity and broaden condition coverage.
Non‑invasive format minimizes risk and improves maternal comfort during testing.
Restraints
Ethical concerns around interpretation and potential misuse of genetic data.
Cost barriers and reimbursement challenges that may limit accessibility in certain regions.
Opportunities
Expansion into developing markets with growing maternal health programs.
Personalized prenatal care, where genetic insights can tailor clinical decisions.
Challenges
Addressing regulatory and ethical frameworks to ensure responsible clinical use.
Balancing cost‑effectiveness with precision, especially in emerging regions.
Regional Analysis
The NIPT market demonstrates varied performance across global regions:
North America
North America holds a dominant share, driven by advanced healthcare infrastructure, favorable reimbursement policies, and high adoption of cutting‑edge genetic technologies.
Europe
Europe follows closely, with widespread awareness and increasing integration of NIPT into standard prenatal care.
Asia‑Pacific
The Asia‑Pacific region is emerging as the fastest‑growing market, propelled by rising healthcare access, awareness campaigns, and expanding prenatal care programs—especially in highly populated countries like India and China.
Latin America & Middle East‑Africa
These regions present significant growth potential due to improving health infrastructure and increasing investment in maternal health screening technologies.
Recent Developments
Major genetic testing firms continue releasing improved NIPT workflows and automation instruments, which streamline cfDNA extraction and analysis, expanding access and efficiency in emerging markets.
Government prenatal care initiatives, such as large‑scale screening programs in countries like India, are boosting overall prenatal testing uptake and awareness of genetic conditions.
Leading market players—including Centogene, Natera, Roche, Illumina, and Quest Diagnostics—are enhancing their product portfolios and expanding service networks to capture growing global demand.
These developments underscore the evolving landscape where innovation, clinical adoption, and policy changes intersect to shape the future of prenatal genomic screening.
Final Thoughts
The Non‑Invasive Prenatal Testing Market is poised for significant expansion due to technological progress, shifting clinical practices, and increased global demand for safer prenatal diagnostics. While challenges around cost, regulation, and ethical use remain, the overall trajectory is highly positive, with strong growth anticipated across all major regions through 2032 and beyond.
To Get Detailed Overview, Contact Us: https://www.acumenresearchandconsulting.com/contact-us