Dual Chamber Prefilled Syringes Market Size
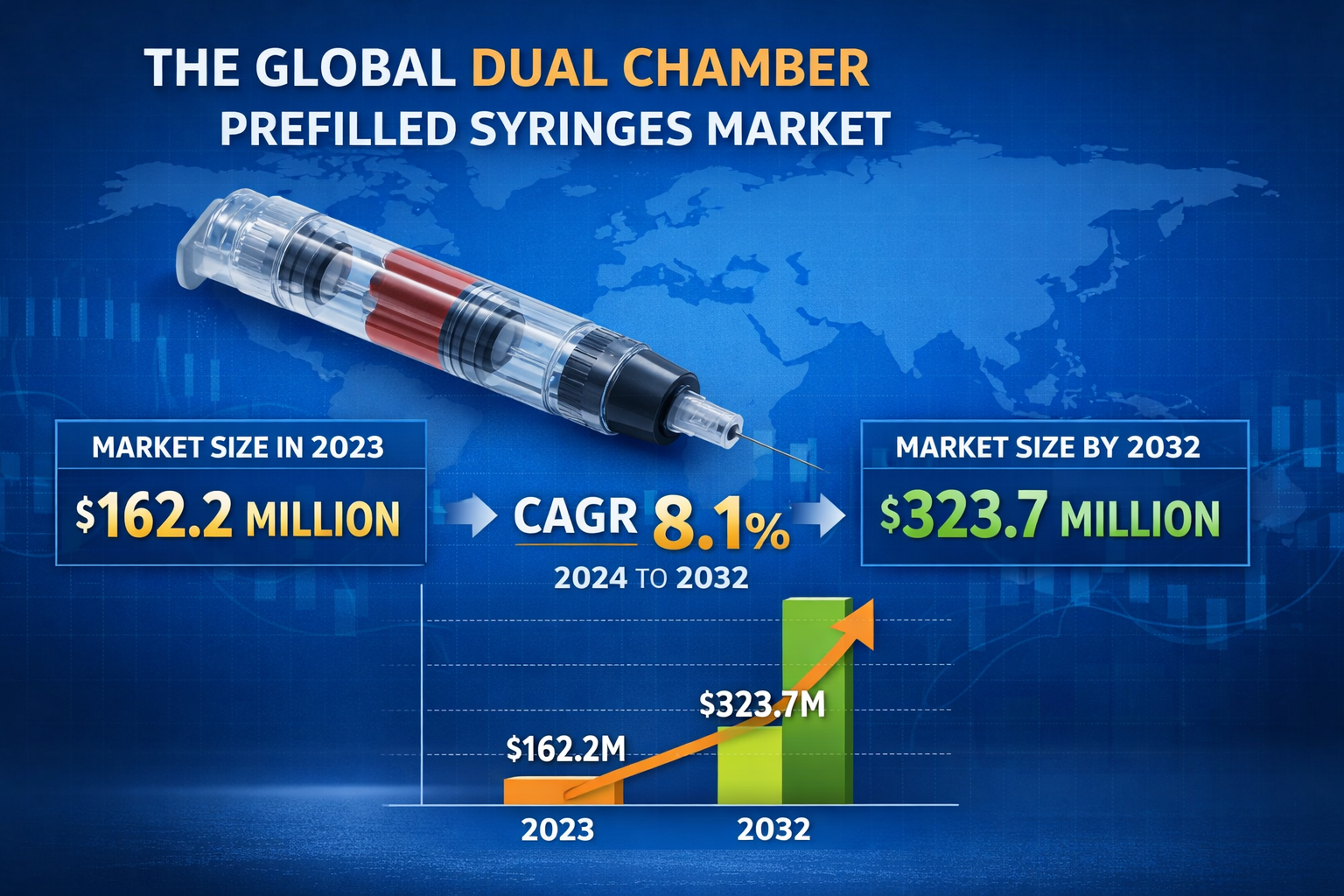
The global dual chamber prefilled syringes market was valued at USD 162.2 million in 2023 and is projected to reach USD 323.7 million by 2032, growing at a CAGR of 8.1% during 2024–2032.
What Is the Dual Chamber Prefilled Syringes Market?
The Dual Chamber Prefilled Syringes Market refers to the global industry centered on medical devices designed with two separate chambers — typically one containing a lyophilized (freeze‑dried) drug and the other containing its diluent (liquid). These chambers remain separate until just prior to injection, ensuring that sensitive medicines are mixed at the point of use for maximum stability, potency, and safety.
Market Trends
The Dual Chamber Prefilled Syringes Market has seen notable trends driven by clinical, technological, and healthcare service shifts:
1. Rising Demand for Biologics and Complex Injectables
As pharmaceutical pipelines increasingly focus on biologics — including monoclonal antibodies and specialty therapies — the need for advanced drug delivery systems like dual chamber syringes has risen. These devices preserve drug integrity until administration, making them indispensable in biologic therapy delivery.
2. Enhanced Safety and Patient‑Friendly Design
Dual chamber syringes significantly reduce the risk of contamination and medication errors by eliminating manual mixing steps. This trend aligns with broader healthcare goals of improving patient safety and reducing preparation errors, particularly in fast‑paced clinical environments.
3. Self‑Administration and Home Healthcare
With rising adoption of self‑administered therapies — particularly for chronic diseases — dual chamber syringes are increasingly used outside traditional clinical settings due to their ease of use and preparation convenience.
4. Material Innovation
Glass syringes currently hold a dominant share due to their chemical inertness and compatibility with biologics. However, advances in polymer materials are paving the way for lightweight, shatter‑resistant alternatives with broader clinical applications.
Get a Free Sample: https://www.acumenresearchandconsulting.com/request-sample/3770
Market Dynamics
Understanding the forces shaping this market explains both its rapid growth and the challenges ahead:
Key Drivers
Growth of Biologics Market: The increasing prevalence of chronic diseases and biologic therapies boosts demand for ready‑to‑use delivery systems.
Focus on Safety and Convenience: Healthcare providers seek delivery formats that improve dosing accuracy and reduce contamination hazards.
Emerging Market Healthcare Investments: Expanding healthcare infrastructure, particularly in Asia‑Pacific and Latin America, offers new adoption opportunities.
Restraints
High Production and Device Costs: Complex design and manufacturing requirements make these syringes more expensive than traditional formats.
Regulatory Complexities: As combination drug–device products, dual chamber syringes face stringent approvals and compliance standards, which can slow time‑to‑market.
Opportunities
Expansion into Emergency Medicine and Chronic Care: These devices are increasingly used in emergency settings and long‑term disease management.
Technological Enhancements: Innovations in materials and device features can improve performance, patient experience, and market penetration.
Challenges
Competition from Alternative Delivery Systems: Despite advantages, dual chamber syringes compete with other delivery formats like autoinjectors and conventional prefilled syringes.
Regional Analysis
Geographical dynamics shape how demand and growth unfold across global markets:
North America
North America currently leads the market, fueled by well‑established healthcare infrastructure, strong pharmaceutical R&D presence, and high adoption rates for advanced injectable systems. Hospitals in the region frequently use these devices for complex therapies, particularly for chronic diseases.
Asia‑Pacific (APAC)
The Asia‑Pacific region exhibits some of the fastest growth potential, with significant investments in healthcare infrastructure and rising awareness of modern drug delivery systems. Expanding pharmaceutical manufacturing and increasing access to biologics contribute to accelerating market expansion.
Europe
Europe’s market growth is supported by aging populations and higher prevalence of chronic conditions, driving demand for reliable and efficient injectable treatments. Strong regulatory frameworks further support adoption of advanced delivery technologies.
Latin America and Middle East & Africa
Although smaller in market size compared to North America and APAC, these regions are showing growing interest as healthcare systems modernize and access to injectable therapies improves.
Recent Developments
Key developments shaping the market include:
Manufacturing Expansion
Significant investments, such as new facilities for glass and polymer syringe production, illustrate industry commitment to scaling manufacturing capacity and supporting demand for advanced syringe formats.
Product Innovation
Specialized polymer‑based syringe systems designed to enhance safety and efficiency are entering the market, indicating a shift toward more advanced device options beyond traditional glass formats.
Regulatory Approvals & New Therapeutics
The approval of new injectable therapies in major healthcare markets stimulates demand for compatible dual chamber systems. Regulatory agencies continue adapting frameworks that support innovative delivery formats through safety and quality standards.
Conclusion
The Dual Chamber Prefilled Syringes Market is positioned for notable growth as the healthcare industry’s needs shift toward safer, more efficient, and patient‑centric drug delivery systems. With strong drivers like the rise of biologics, expanding self‑administration trends, and evolving device technologies, the market is expected to nearly double by 2032. While challenges around cost and regulation persist, regional investments and innovation present robust opportunities for manufacturers, healthcare providers, and investors alike.
To Get Detailed Overview, Contact Us: https://www.acumenresearchandconsulting.com/contact-us