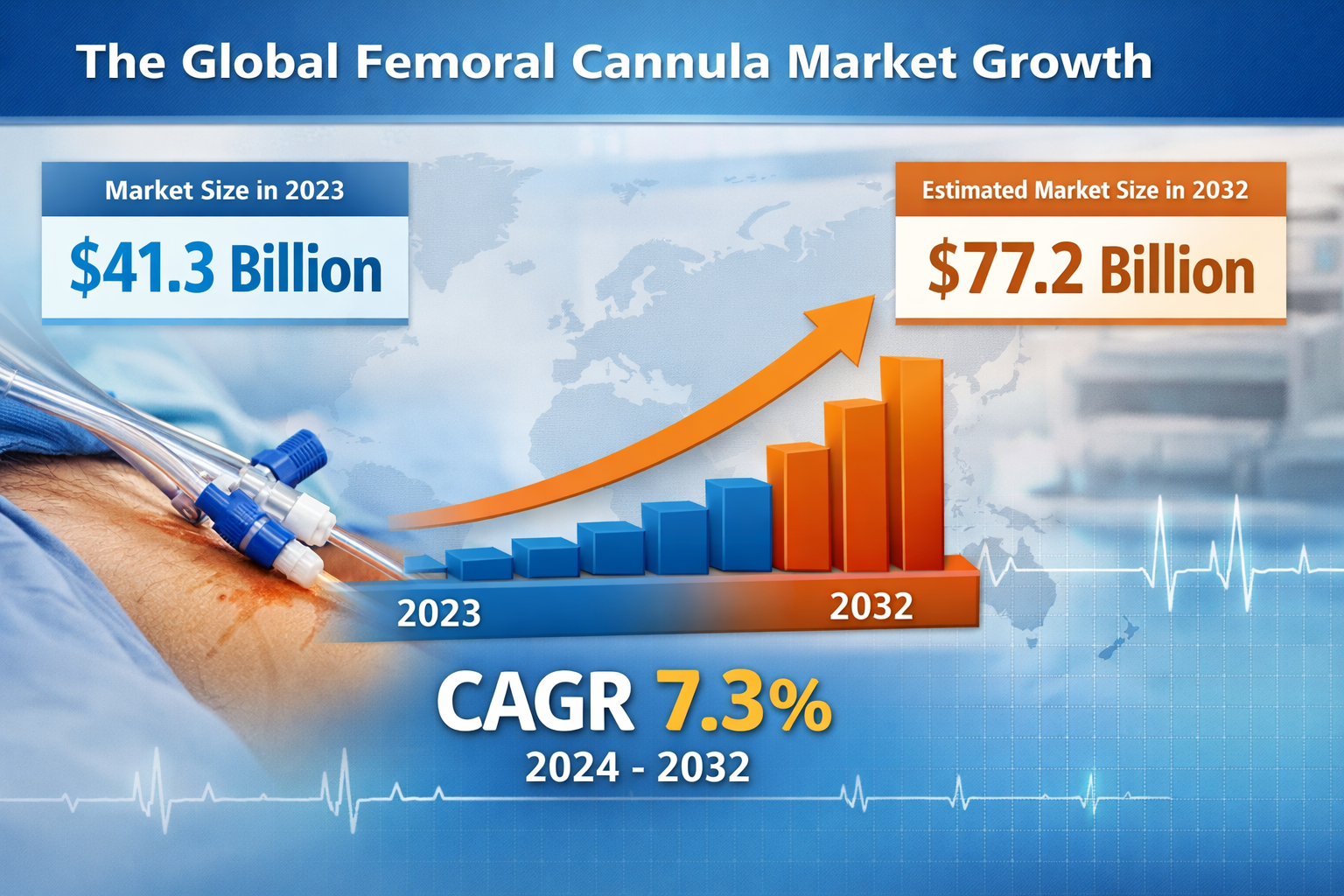
Femoral Cannula Market Size
The global femoral cannula market was valued at approximately USD 41.3 billion in 2023 and projected to reach USD 77.2 billion by 2032, growing at a CAGR of 7.3%.
What is the Femoral Cannula Market?
The Femoral Cannula Market refers to the global industry involved in manufacturing, distributing, and innovating femoral cannula devices—tubular instruments used to access the femoral artery or femoral vein during critical medical procedures. These cannulas enable blood to flow outside the body and into mechanical support systems such as extracorporeal membrane oxygenation (ECMO) or cardiopulmonary bypass during surgeries and intensive care interventions.
Scope & Significance
Applications: Cardiac surgical interventions, hemodialysis access, cardiopulmonary resuscitation, hemoperfusion access, ECMO support, extracorporeal life support, and other emergency procedures.
End‑Use Channels: Hospitals, clinics, dialysis centers, cardiac surgical centers, and ambulatory surgery centers.
The increasing prevalence of cardiovascular diseases, coupled with wider adoption of advanced surgical procedures, reinforces the importance of femoral cannulas in modern healthcare systems globally.
Get a Free Sample: https://www.acumenresearchandconsulting.com/request-sample/3715
Market Trends
1. Technological Advancements
Innovation in femoral cannula design is a key trend shaping the market:
Biocompatible Materials: Modern cannulas use advanced materials to minimize immune response and reduce complications.
Improved Safety Features: Anti‑thrombogenic coatings and ergonomic designs enhance patient safety and procedural efficiency.
Minimally Invasive Approaches: A notable shift toward less invasive procedures has increased cannula adoption in cardiovascular and vascular surgeries.
2. Increasing Adoption in Emergency & Critical Care
Hospitals and critical care units are increasingly deploying femoral cannulas due to their rapid access and versatility, particularly important in ECMO and cardiopulmonary bypass cases.
3. Growth in Life‑Saving Procedures
Demand for life‑supporting treatments, especially in managing respiratory or cardiac failure, continues to drive cannula usage worldwide, given their pivotal role in ECMO support and open‑heart surgeries.
Overall, trends point toward more advanced, patient‑centric designs and wider clinical adoption across emergency and surgical settings.
Market Dynamics
Understanding the forces shaping this market helps industry players and stakeholders strategize for future growth.
Drivers
Rising Prevalence of Cardiovascular Disorders: Heart disease remains a leading cause of mortality, increasing reliance on surgical and life‑support procedures.
Higher ECMO & Cardiopulmonary Bypass Adoption: These procedures rely on femoral cannulas for effective blood circulation support.
Innovations in Device Design: Continual enhancements in device safety and performance spur broader usage.
Restraints
High Device Costs: Advanced cannulas often carry significant cost implications for healthcare providers.
Risk of Complications: Improper insertion can lead to vascular injury or infection, which limits use in some regions.
Need for Skilled Professionals: Complex procedures require trained medical staff, which can slow adoption in resource‑limited regions.
Opportunities
Expansion in Emerging Markets: Growing healthcare infrastructure in Asia‑Pacific and Latin America offers substantial growth potential.
Minimally Invasive Surgical Growth: Rising preference for less invasive interventions boosts demand for advanced cannula systems.
R&D Focus: Increased investment in research encourages development of safer and more effective cannulas.
Regional Analysis
Regional performance varies based on healthcare infrastructure, investments, and clinical adoption rates.
North America
Dominant Market Position: In 2023, North America led revenue generation.
Driving Factors: Advanced healthcare systems, higher prevalence of cardiovascular diseases, and greater procedural volumes.
Asia‑Pacific
Fastest Growing Region: Expected to grow at a CAGR above 8% during the forecast period.
Growth Catalysts: Expanding healthcare infrastructure in countries like India, China, and Japan, combined with rising awareness of advanced treatments.
Europe, Latin America, Middle East & Africa
Europe maintains steady growth due to established healthcare systems, while Latin America and the Middle East & Africa represent emerging markets with increasing investments in medical infrastructure.
Recent Developments
While detailed mergers or regulatory changes are not highlighted, key market developments include:
Strategic Portfolio Expansion: Leading manufacturers are engaging in product launches, collaborations, and portfolio expansion initiatives.
Innovation in Products: Companies are developing new cannula variations optimized for different clinical needs, including pediatric and specialized applications.
Collaborative Efforts: Partnerships between device makers and healthcare institutions are becoming more prevalent, enabling improved product development and clinical evidence support.
Conclusion
The Femoral Cannula Market stands at a pivotal inflection point where rising clinical demand meets technological progress. Fueled by the prevalence of cardiovascular diseases, growing adoption of critical care procedures like ECMO, and innovations in device design, this market is poised for robust growth through 2032 and beyond. Emerging regional markets and expansion of minimally invasive surgical practices further broaden opportunities. While challenges such as cost and training requirements persist, advancing cannula technology and expanding healthcare access position the industry for sustained evolution.
To Get Detailed Overview, Contact Us: https://www.acumenresearchandconsulting.com/contact-us